In an online resource like this it isn’t really possible to do justice to the complexity and depth of a subject as broad as the immune system. Instead what we try to provide here is a simple guide to the basic players in the different arms of the immune system.
The first line of defence against pathogens is the physical barriers of the skin and mucous membranes. Any invader that breaches this physical barrier is then met by the innate immune system. It is referred to as innate because all animals naturally have it. In fact, parts of the innate system evolved over 500 million years ago. The innate immune system plays a critical role as an ultra-fast second line of defence that can often handle an infection without any further support. However, when required the innate immune system also acts as an activator and controller of the third line of defence, the adaptive immune system. All vertebrates have an adaptive immune system and as the name suggests, this part of the immune system can adapt to almost any invader.

The immune system is governed by a group of cells known collectively as white blood cells or leukocytes. Here we summarise the main cells and their activities in both the innate and adaptive immune systems. Leukocytes can be classified as granulocytes or agranulocytes based on the presence or absence of microscopic granules in their cytoplasm when stained.
Granulocytes consist of neutrophils, basophils, eosinophils and mast cells.
Agranulocytes can be classified as monocytes or lymphocytes. Monocytes are the largest leukocytes, have a nucleus that lacks lobes and are effective phagocytes. When monocytes leave the bloodstream and enter the tissue they differentiate into tissue-specific phagocytes called macrophages and dendritic cells. Macrophages in specific body tissues develop characteristics suited to the particular tissue. Not only do they provide immune protection for the tissue in which they reside but they also support normal function of their neighboring tissue cells through the production of cytokines. Macrophages are given tissue-specific names, and a few examples of tissue-specific macrophages are microglial cells (brain and CNS), Kupffer cells (liver) and alveolar macrophages (lungs).Dendritic cells are important sentinels residing in the skin and mucous membranes. They act as important phagocytes and antigen presenting cells.
Lymphocytes determine the specificity of the immune system and account for 20-40% of all leukocytes. They are found in the circulation and concentrated in the spleen, tonsils and lymph nodes. The two primary types of lymphocytes are B cells, which mature in the bone marrow, and T cells, which mature in the thymus. As will be discussed in the section on adaptive immunity, B cells can differentiate into plasma cells, which are antibody factories, or memory B cells, which provide long term immunity. T cells differentiate into cytotoxic T cells, which kill infected cells, T helper cells, which are cytokine factories, regulatory T cells, which keep the immune system from over-reacting and memory T cells.
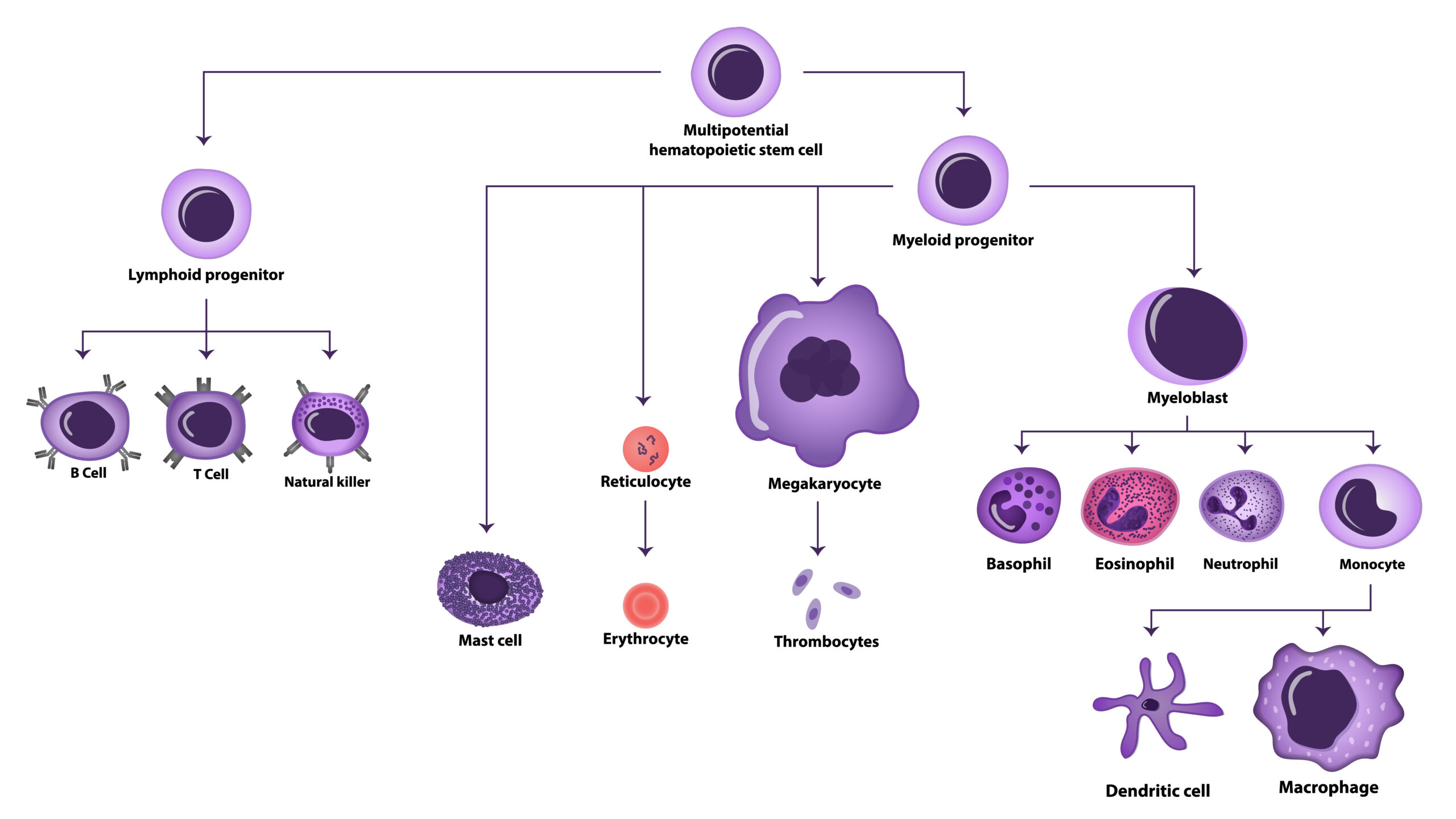
Figure 1. Schematic representation of Hematopoiesis and the formation of blood cells.

In the sections on innate and adaptive immunity we will touch on the topics of cells presenting antigens to other cells and cells assessing the health of other cells but how does this process work? Well the presenting is done by special proteins called major histocompatibility complex (MHC) proteins, of which there are two types. MHC class I molecules are found on the surface of most cells. These present peptides from inside the cell onto the surface of the cell and thus enable immune cells, particularly cytotoxic T cells, to ‘see’ what is going on inside the cell and get an indication of its health.
MHC class II molecules are only expressed on the surface of antigen presenting cells, such as macrophages. These act to inform helper T cells of on going infections. For instance, during a bacterial infection a macrophage will phagocytose a bacterium and load fragments of bacterial proteins onto MHC class II molecules for display on its surface. Helper T cells will then scan the surface of the macrophage using their T cell receptors.
So, in short, class I MHC molecules alert cytotoxic T cells when something isn’t right inside a cell, and class II MHC molecules inform helper T cells when a problem exists outside of cells.

The innate immune system is a hard-wired defence that has evolved to recognize the hallmarks of pathogens that commonly cause infection. There are numerous systems at play that interact, cooperate, regulate and activate each other to ensure a rapid and proportional response.
The complement system
In very basic terms the complement system is a pathway of about 20 different proteins that work together to destroy pathogens and activate other parts of the immune system. The proteins of the complement pathway are produced primarily by the liver and are present at high concentrations in the blood and tissues. The most abundant protein in the pathway is C3 and this spontaneously breaks down into two fragments, C3a and C3b. C3b is highly reactive and binds to either amino or hydroxyl groups, which are common on the surface of pathogens as well as host cells. C3b is extremely short lived and is rapidly neutralized by binding to a water molecule. This means that C3b has to be very close to a target to react. Once it reacts with an appropriate chemical group it is stabilized on the cell surface and binds to another complement protein, B. This C3bB complex is then cleaved by complement protein B to yield C3bBb. This molecule then setups a positive feedback loop as C3bBb acts as a convertase to greatly enhance the fragmentation of C3 into C3a and C3b. This process generates ever more C3b which can attach to the cell surface and form C3bBb. The C3bBb can bind another molecule of C3b and together they clip the complement protein C5 into C5a and C5b. C5b then combines with C6, C7, C8 and C9 to form a membrane attack complex (MAC), which opens up a hole in the surface of the target leading to cell lysis.
Complement can efficiently create holes in bacteria, parasites and some enveloped viruses but also healthy tissue – in fact this is one of the causes of transplant rejection. To prevent the complement system from attacking the hosts own cells there are numerous safeguards in place. Complement protein C3b can be converted to an inactive form by proteins in the blood and this is further accelerated by membrane cofactor protein (MCP or CD46) that is present on the surface of cells. Another protein, decay accelerating protein (DAF or CD55), accelerates the destruction of the convertase C3bBb by other blood proteins and thus can prevent the positive feedback loop from starting. A further protein, protectin (CD59), prevents the incorporation of C9 molecules into the MAC.
The default mode for the complement system is target death. If a cell surface is not protected from complement it will be attacked extremely rapidly. The complement system can be activated in 3 ways. All three paths follow the same process from the C3 convertase, they just differ in how this is activated. The first is the alternative pathway, which is the spontaneous cleavage of C3 to produce C3b. This is always on but at a low rate until the cleavage of C3 is accelerated by a feedback loop. The second is the classical pathway, so called because it was discovered before the alternative pathway. This depends on antibody activation and so will be covered in the section on the adaptive immune system. The third is the lectin activation pathway.
The main protein in the lectin activation pathway is mannose-binding lectin (MBL). Lectins are proteins that are able to bind carbohydrate molecules and mannose is found on the surface of many pathogens but not healthy cells. In the blood MBL binds to mannose associated serine protease (MASP) which functions like a convertase to clip C3 to form C3b, thus initiating the complement chain reaction.
In addition to building MACs the complement system has two other important functions. When C3b is attached to a pathogen it can be clipped by complement factor I to produce an inactive form known as ic3b. Rather than playing no further role in host defence the ic3b can opsonize a pathogen much in the same way as an antibody. Phagocytes, such as macrophages, express complement receptor type 2 (CR2 or CD21) and CR3 (CD11b/CD18), which bind to iC3b and facilitate phagocytosis.
Finally, the complement system also plays a role by attracting immune cells to the site of the pathogen. Many of the fragments of complement proteins that are created during the aforementioned cascade, such as C3a and C5a, are highly potent chemoattractants. C5a, for example, is an especially powerful chemoattractant for macrophages.
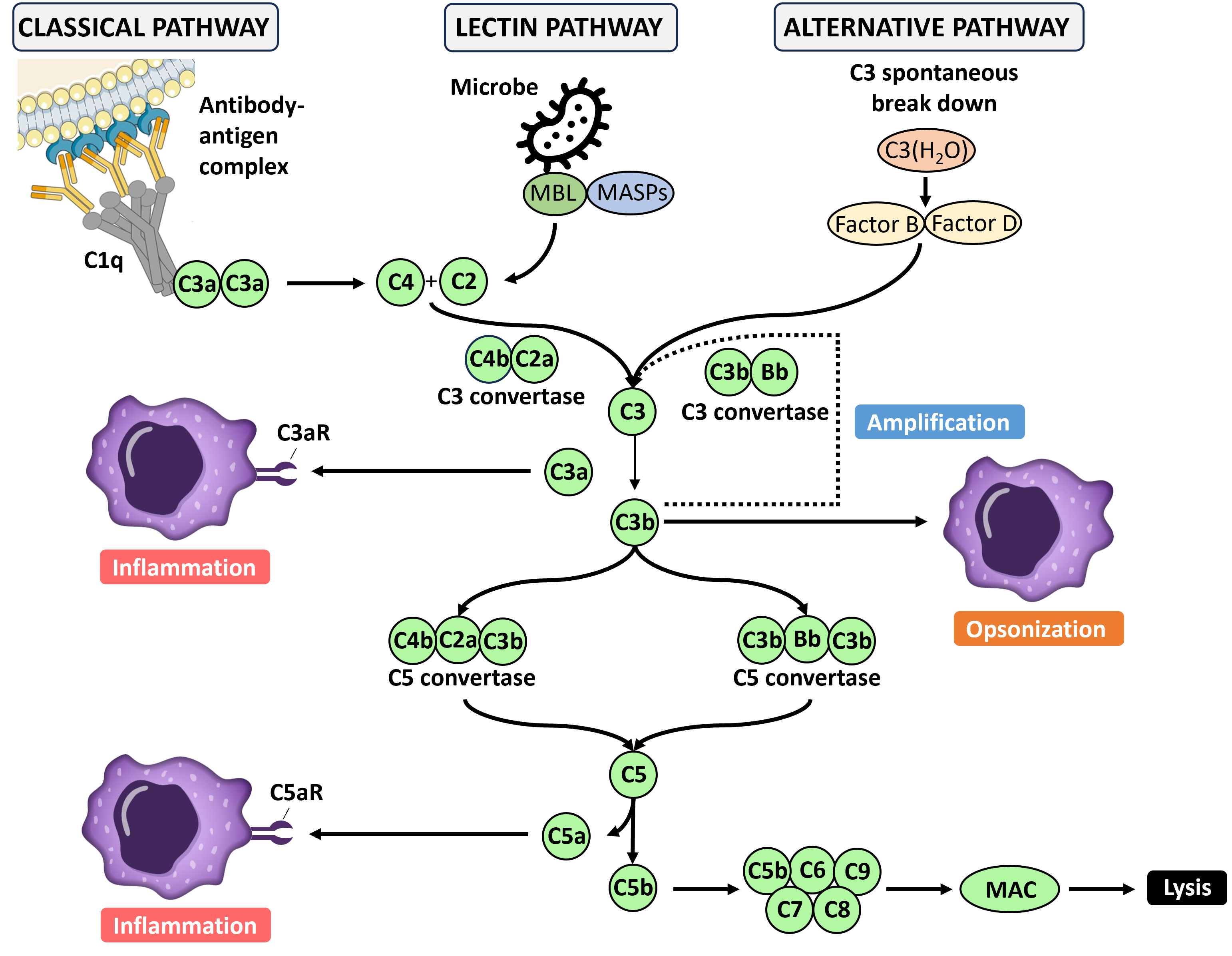
Figure 2. The complement cascade.
Phagocytes
The second arm of the innate system are cells collectively known as phagocytes, which primarily function to ‘eat’ or phagocytose other cells, debris or foreign material. The two most important phagocytes are macrophages and neutrophils.
Macrophages are found just under the surface of most areas of the body that are exposed to the outside world, i.e. those spaces that are most susceptible to microbial infection. Macrophages exist in what are classically defined as three states of readiness (although these are actually a continuum): resting; primed (or activated); and hyperactivated. In the resting state they act to clean tissues up from dead cells. When macrophages receive signals that alert them to the presence of a pathogen they become activated or primed. In this state macrophages increase their rate of phagocytosis and upregulate expression of MHC class II molecules so they cannot act as antigen presenting cells (APCs). A number of different signals can prime a resting macrophage but the most well-known is the cytokine interferon gamma (IFN-γ). This cytokine is produced mainly by helper T cells and NK cells. In the primed state, macrophages are good APCs and reasonably good killers. However, there is a higher state of readiness, known as hyperactivation, which can be achieved when the cell receives a direct signal from a pathogen. Macrophages express receptors for ‘danger signals’ such as lipopolysaccharide (LPS) or mannose. When any danger signals are detected a macrophage stops proliferating and focuses on killing. They grow very large and increase their rate of phagocytosis. They also produce the cytokine tumor necrosis factor (TNF). This cytokine can kill tumor cells and virus infected cells as well as helping to activate other immune cells.
Macrophages are extremely versatile and can handle minor infections alone but for larger infections they require support, namely in the form of the neutrophils. Unlike macrophages, neutrophils are very short lived (approximately 5 days), are present primarily in the blood and are not antigen presenting cells. Once summoned to a site of infection it takes neutrophils about 30 minutes to exit the blood and become fully activated. In this state they are incredibly phagocytic. Neutrophils also produce cytokines, such as TNF, to alert other immune cells. Most importantly, activated neutrophils secrete destructive chemicals that turns tissue into a toxic soup that is lethal to invading microbes. They are the only immune system cell capable of liquifying both cells and connective tissue and for this reason are short-lived to limit collateral damage. As neutrophils represent about 70% of all circulating white blood cells there are always more on call if needed.
Phagocytes and other immune cells recognise so called danger signals via pattern-recognition receptors (PRRs), of which there are more than 20 types. PRRs can be broadly classified into two groups, those that detect pathogen associated molecular patterns (PAMPs) and those that recognize damage associated molecular patterns (DAMPs).
Molecules that function as PAMPs are typically structural features that are critical to the pathogen and so cannot be easily mutated to avoid detection. One of the most well classified PRRs are the Toll-like receptors (TLRs). There are 10 human TLRs with different cells expressing different combinations of these. Some TLRs are displayed on the cell surface, such as TLR4 which detects LPS, while others are intracellular, like TLR7 which detects single stranded RNA of viruses and TLR9 which recognises double stranded DNA of bacteria and herpes simplex virus.
Molecules that function as DAMPs are normally intracellular and are released by dying cells. In this way DAMPs can alert the immune system to widespread cellular death associated with an infection. This is important as it allows the immune system to respond to pathogens for which there are no PRRs.
The interferon system
When PRRs recognize a viral attack they produce interferon alpha (IFN-α) and interferon beta (IFN-β). As their name suggests, these type I interferons interfere with viral reproduction. Most cells quickly produce type I interferons when attacked by a virus and most have receptors on their cell surface to detect these proteins. When type I interferons bind to a cell which is itself infected this upregulates the production of several hundred different anti-viral proteins that act to reduce the amount of virus produced by the infected cell. Additionally, type I interferons also act as warning signals to other nearby uninfected cells. The alerted cells switch on the expression of anti-viral genes and prepare to commit suicide if they become infected.
Natural killer cells
Another important player in the defence against viral infection is the natural killer (NK) cell. NK cells are typically found in the blood, spleen or liver and are typically shot lived with a half-life of about a week. Once called to the tissue due to an infection they rapidly proliferate to increase their numbers and then perform two main functions. Firstly they secrete IFN-γ, which activates other cells, such as macrophages. Secondly, they destroy virus-infected cells by forcing them to commit suicide. In some cases NK cells use perforin protein to inject suicide enzymes, such as granzyme B, into a target cell. In other situations Fas ligand on the NK cell surface interacts with Fas on the surface of the target cell which signals for self-destruction.
Like other immune cells, NK cells can be hyperactivated, making them more efficient killers and secreters of cytokines. During a viral attack, NK cells are hyperactivated by IFN-α or IFN-β secreted by virus-infected cells. During a bacterial infection NK cells can by hyperactivated by LPS.
NK cells recognise their target using activating and inhibiting receptors on their cell surface. Inhibitory receptors recognize MHC class I molecules on the surface of the target cell and lead to a “don’t kill” signal. Activating receptors lead to a “kill signal” by interacting with unusual carbohydrates or proteins on the surface of the target cell which act as warning flags to indicate the cell has been infected or is becoming cancerous. In this way NK cells measure the health of a cell by the relative strength of the kill or don’t kill signals and thus determine whether a cell should be destroyed or not.

As its name suggests, the adaptive immune system is capable of protecting us by adapting to almost any invader. One of the earliest and most famous demonstrations of this was Edward Jenner’s inoculation of a small boy with the deadly disease smallpox having previously inoculated the child with the much milder but very highly related cowpox virus. This early demonstration of vaccination gave the child protection against cowpox and the closely related smallpox but not other viruses, i.e. the immune system had adapted to very specifically deal with one particular type of virus.
Antibodies and B cells:
Immunologists later discovered that this immunity was driven by antibodies. There are five classes of antibody: IgA, IgD, IgE, IgG and IgM. They all have the same basic structure consisting of two heavy and two light chains forming two Fab arms containing identical domains at either end attached by a flexible hinge region to the stem of the antibody, the Fc domain, giving the classical ‘Y’ shape. In very basic terms, the Fab arms bind to the target antigen, which can be almost anything, and the Fc domain interacts with cells of the immune system. All antibodies are produced in B cells, a type of leukocyte produced in the bone marrow (hence the ‘B’), which mature into antibody factories known as plasma B cells. The diversity of antibodies is enormous. Immunologists estimate there are about 100 million different antibodies. This diversity is created through processes known as VJD recombination and junctional diversity, which we won’t go into here.
There are about three billion B cells in the bloodstream, which may seem a lot but this means that on average there are only about 30 B cells to any given target. Instead, B cells are made on demand in a process known as clonal selection. Each B cell expresses membrane bound versions of the antibody on its cell surface as B cell receptors (BCRs). All BCRs on a given B cell recognise the identical antigen. The B cells then essentially fish for their cognate antigen. When a B cell binds its cognate antigen it is triggered to proliferate. This process lasts about a week with each cell growth and division cycle taking about 12 hours. Over this time period a single cell becomes a clone of approximately 10,000 identical B cells. After this period of proliferation the B cells switch to secrete soluble forms of the antibody into the blood stream for about a week before then dying.
Interestingly, antibodies don’t actually kill anything themselves, they simply tag things for destruction by other components of the immune system. There are three main ways this can happen: antibody dependent cellular cytotoxicity (ADCC); antibody dependent cellular phagocytosis (ADCP); and complement dependent cytotoxicity (CDC).
ADCC is the mechanism in which certain immune cells, particularly natural killer (NK) cells, recognize and eliminate target cells marked with specific antibodies. The process begins when antibodies bind to antigens on the surface of target cells, forming immune complexes. Fc receptors on the surface of NK cells then recognize the Fc domain of these antibodies, triggering the release of cytotoxic substances and leading to the destruction of the target cell.
ADCP is the mechanism in which phagocytic cells, such as macrophages and neutrophils, are activated to engulf and digest target cells or pathogens that have been tagged with specific antibodies. The process initiates when antibodies bind to antigens on the surface of the target cell, forming immune complexes. Fc receptors on the surface of phagocytic cells recognize the Fc domain of these antibodies, triggering the phagocytic cell to engulf the antibody-coated (opsonized) target. Once internalized, the phagosome containing the target is fused with lysosomes, leading to the degradation and elimination of the target.
CDC is the mechanism by which antibody clustering on a target surface initiates the classical arm of the complement pathway through the initial multivalent binding of C1q to the antibody Fc domains. As described above, this cascade ends in the formation of a membrane attack complex and the lysis of the target cell.

Figure 3. The biological functions of the generic Fc gamma receptor.
Alternatively, and specifically for viruses, antibodies can function simply as neutralizing agents. Here the antibodies bind to an epitope on the virus that is required for docking to cells. Although this does not destroy the virus it does prevent it from entering the cell and reproducing, thus neutralizing the threat.
T cells:
Once a virus gets into a cell, antibodies can’t get to it. This is where T cells come in. T cells are very similar to B cells in appearance but rather than BCRs they display T cell receptors (TCRs) on their surface. TCRs are about as diverse as BCRs and T cells employ a process of clonal selection like B cells. So, like the antibody response, the T cell response is slow and specific. Although there are many similarities between B and T cells, there are also some key differences. B cells make antibodies that can recognise any organic molecule but T cells specialise in recognizing protein antigens. A B plasma cell secretes antibodies but a T cells receptors always remain bound to the cell surface. Lastly, a B cell can recognize an antigen by itself but a T cell only recognizes antigen if it is properly presented by another cell.
There are four types of T cells: killer T cells (also called cytotoxic lymphocytes or CTLs); helper T cells; regulatory T cells; and memory T cells. CTLs are very potent killers of virus infected cells by triggering cells to commit suicide. Helper T cells direct the immune response by secreting cytokines. Regulatory T cells act to prevent the immune system from over-reacting or reacting inappropriately, while memory T cells provide long lasting immunity.
Immunological memory:
After B and T cells have been activated, proliferated and dealt with the immediate threat, most of them die off. However, a small number of them remain as memory cells. These are more numerous than the original naïve B or T cells and they are also easier to activate. This means that during a second exposure to the same pathogen the adaptive immune system can spring into action extremely quickly, often to the point that you never experience any symptoms.
Tolerance:
As described earlier, B and T cell receptors are so diverse they can recognise anything. This includes recognising our own molecules, which if it happened would result in our immune system attacking our own bodies, known as autoimmunity. To avoid this scenario B and T cells are screened to prevent autoimmunity.

The topic of immunology is so broad and deep that it is impossible to cite papers that give a useful introduction to the topic. Instead we recommend the below books which all give basic introductions to the topic. All these books are available to purchase on Amazon at a reasonable price.
1. Playfair, J. H. L. & Chain, B. M. Immunology at a Glance. (Wiley-Blackwell, 2012).
2. Klenerman, P. The Immune System: A Very Short Introduction. (Oxford University Press, 2018).
3. Sompayrac, L. M. How the Immune System Works. (Wiley-Blackwell, 2022).
4. MBBS, A. K. A., PhD, A. H. L. M. & PhD, S. P. M. Basic Immunology: Functions and Disorders of the Immune System. (Elsevier, 2023).