In 1958 F. W. Rogers Brambell investigated the passage of maternal antibodies to fetuses and neonates, which led to him proposing the idea of a receptor that interacted with the Fc domain of IgG (1). However, it took almost 30 years before the receptor regulating IgG transport, known as the neonatal Fc receptor (FcRn) or Brambell receptor, was isolated and characterized (2). FcRn was classified as a non-classical Fc gamma receptor (FcγR) as it was structurally unique and not thought to be involved in the immune response. Unlike classical FcγRs, FcRn is a beta-2-microglobulin (β2M) associated protein. It is structurally related to the major histocompatibility class I (MHC-I) family but is unable to present peptides to T cells (3).
FcRn is expressed in most cell types and is predominantly intracellular. In addition to binding IgG it also binds to albumin (4). These two proteins owe their long half-life to this interaction with FcRn. FcRn is also responsible for the transport of IgG from mother to offspring, providing the naïve and immature immune system of the child with the experience and protection developed by the mother. The functions of FcRn are determined by whether IgG is a single monomeric molecule or an immune complex. As an immune complex FcRn has been shown to regulate the immune response as well as processing and presentation of antigens within the immune complex. Although FcRn is typically considered as the transport molecule, additional roles beyond this are still being elucidated (5).

FcRn is structurally related to MHC-I molecules with a 40 kDa alpha (α) heavy chain the non-covalently associates with the 12 kDa light chain β2M (6-8). The heavy chain consists of 3 extracellular domains, a transmembrane domain and a short cytoplasmic tail (8). The typical peptide binding groove found on MHC-I molecules is occluded, thus preventing FcRn from binding and presenting peptides (6). Instead FcRn binds with high affinity to IgG and albumin through non-overlapping sites at mildly acidic pH 5.0 to 6.5 with no detectable binding at neutral pH. The exceptions to this are mouse IgG2b and human IgG3 which display weak binding at neutral pH to mouse and human FcRn respectively and thus have reduced half-lives compared to other IgG sub-types (9, 10).
FcRn interaction with the Fc domain of IgG occurs at the interface of the CH2 and CH3 domains, which is distinct from the binding site for classical FcγRs in the hinge and upper CH2 region. Of particular importance on the Fc domain are two histidines, H310 and H435. Their protonation at pH6 allows for interaction with E115 and D130 on FcRn. As the pH increases above 6, histidine protonation is gradually lost resulting in pH dependent binding (11, 12). As IgG is a homodimeric molecule and thus has two FcRn binding sites, functional interaction of monomeric IgG with FcRn is thought to occur in a 2:1 stoichiometry (13).
Although receptor interactions with the Fc domain have typically been considered to be independent of the Fab domains, recent work has suggested a role for the Fab domains in FcRn binding. Although surface plasmon resonance (SPR) studies have been unable to detect differences in binding for different antibodies (13), a number of other studies have suggested that the charge distribution and isoelectric point (pI) of the Fab domains can affect the dissociation from FcRn at neutral pH and thus the circulating half-life of the IgG (14-18).
Compared to IgG, binding of FcRn to albumin occurs at the opposite side of FcRn and involves a larger more hydrophobic binding surface (4, 19). This interaction also relies on key histidine residues to bestow pH dependency. H166 of human FcRn and H464, H510 and H535 of albumin are critical for binding and mutation of any of these sites reduces binding (20). FcRn contact sites on albumin are also binding sites for albumin cargo, such as fatty acids, thyroxine and various drugs (21) and thus albumin molecules carrying these cargo may exhibit reduced binding to FcRn (19).
Various studies have demonstrated that FcRn can engage with both it its ligands simultaneously, as shown by the co-crystal structure in figure 1 (22, 23). The binding affinities reported for these interactions vary wildly depending on the experimental equipment, setup, reagents and ligands. SPR studies of the human FcRn-albumin interaction at acidic pH report a KD of approximately 1 μM when the receptor is immobilized and 0.2 μM when albumin is immobilized (23, 24, 25). For the human-IgG-FcRn interaction affinities of 0.2-2.3 μM are reported when IgG is immobilized and 10-100 nM when FcRn is immobilized (13, 26-29). The latter values are likely due to avidity with IgG containing two FcRn binding sites. A recent solution-based study comparing albumin and IgG binding to FcRn using microscale thermophoresis (MST) gave KDs of 0.9 and 0.5 μM respectively (30). It should be noted that diversity in binding affinities between FcRn and IgG or albumin are observed across different species (9, 29, 31), making extrapolation from animal models to man challenging.

Figure 1. Co-crystal structure (PDB: 4N0U) of FcRn in complex with an IgG Fc domain and human serum albumin. The FcRn heterodimer is shown in green (FCGRT) and grey (B2M). One half of the IgG Fc domain is shown in blue with albumin shown in pink, cyan and gold for domains I, II and III.

FcRn is a homeostatic regulator of circulating levels of IgG and albumin. The normal half-life of these molecules is 19-21 days compared to a typical half-life of 5-7 days for most other serum proteins larger than the glomerular filtration limit (32). The mechanism underlying this is the pH dependent binding to FcRn and resulting diversion from intracellular degradation pathways, as shown in figure 2. IgG enters endothelial cells non-specifically by pinocytosis and subsequently binds to FcRn in EEA1-, Rab5- and Rab11+ sorting endosomes characterized by a pH of 6. IgG bound FcRn then separates from sorting endosomes to Rab4- and Rab11a+ recycling endosomes, while anything unbound to FcRn is instead sorted to lysosomes (33, 34). Intracellular trafficking studies have shown that while monomeric IgG and small IgG immune complexes are recycled, large IgG immune complexes are predominantly sorted to the lysosome (35). Albumin recycling is thought to occur through a similar process but as the majority of trafficking studies have used IgG as a ligand the specific mechanisms underlying albumin recycling are unclear.
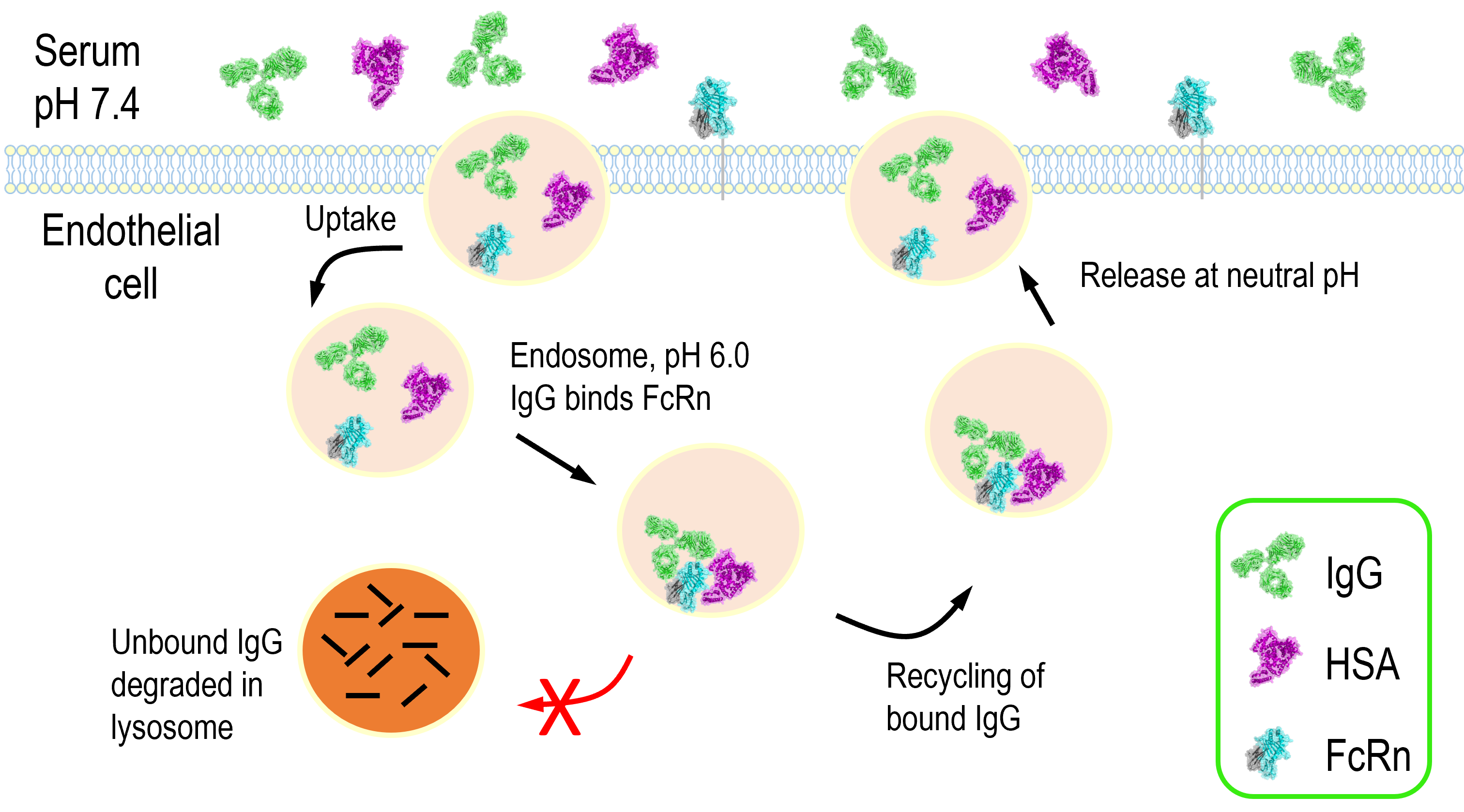
Figure 2. FcRn mediated recycling of IgG and albumin. In the early endosome IgG and/or albumin interacts with FcRn at pH 6.0. The FcRn complex is then recycled back to the cell surface and IgG and albumin are released at neutral pH thus rescuing them from lysosomal degradation.

FcRn expression has been detected almost ubiquitously in diverse tissues including epithelia, endothelia and hematopoietic cells. Below are some of the emerging roles of FcRn in different tissues.
FcRn in the intestine
More than 40 years ago Jones (36) and Rodewald (37) described age and tissue specific transfer of IgG in rodents by illustrating that segments of the proximal jejunum but not ileum of 10-14 day old rats transported only IgG from the lumen to the circulation, which was not detectable in 22 day old rats. Subsequently the receptor responsible for this transport was isolated from neonatal rats (2). Subsequently studies in humans have characterized FcRn expression at intestinal mucosal surfaces throughout life in both the small and large intestine. In humans little maternal IgG is transmitted to the neonatal circulation across the intestines. In contrast, in other species, most notably cattle and pigs, neonates rely entirely on postnatal uptake of colostral antibodies via intestinal epithelium for systemic humoral immune protection (38). Despite these species differences it is clear that FcRn consistently plays a critical role in establishing humoral immunity in mammalian offspring. However, its utility in adults to justify life-long expression is less well understood. IgG is present in mucosal secretions of the gastrointestinal, respiratory nd genital tract where IgG, IgA and IgM function together in host defence (39). However, while IgA and IgM are transcytosed unidirectionally via the polymorphic immunoglobulin receptor, FcRn expressed in epithelial cells mediates IgG transcytosis in both directions (40-44). Thus, FcRn in the intestine can deliver monomeric IgG or IgG immune complex into the lumen or in the reverse direction into the lamina propria. This process ensures specific delivery of luminal antigens in the form of IgG immune complex to mucosal dendritic cells that can then regulate immune responses (42, 43). In fact, the absence of FcRn results in greater susceptibility to mucosal infections (43, 45, 46).
FcRn in the placenta
In some species, notably humans and rats, epithelial transfer of IgG plays little to no role in establishing humoral immunity and instead this occurs via prenatal transfer of IgG across the placenta or yolk sac (47). IgG is the only antibody that is transported across the placenta (48, 49) and this is due to FcRn binding. Of the four subclasses of IgG, IgG1 and IgG4 are transported readily, whereas IgG2 and IgG3 show less efficient transplacental passage (10, 49, 50). Transport of albumin across the placenta does not seem to occur (51). The mechanism behind this is not understood but it has been suggested that other albumin receptors, such as megalin and cubilin, which are expressed in the placenta, might facilitate retrograde recycling of albumin back to the maternal circulation (52).
FcRn in the kidney
FcRn expression in the kidney plays a number of roles. Podocytes have been shown to express FcRn (53) and can transcytose IgG from the filtration membrane to the urinary tract (54). It is believed this clears IgG and IgG immune complexes from the filtration membrane and also provides protective IgG to the urinary tract. FcRn in the kidney is also important for albumin homeostasis (55). The proximal tubule epithelial cells are involved in reabsorption of proteins from the filtrate. FcRn in these cells have been shown to be involved in reabsorption of albumin and potentially IgG (56, 57).
FcRn in the liver
The liver is a major site of FcRn expression with it being detected in liver endothelium, sinusoidal epithelial cells, Kupffer cells, hepatocytes and biliary epithelium (58-60). The contribution of hepatic FcRn to IgG or albumin biology is still emerging but seems to serve two main purposes: maintenance of monomeric IgG and albumin in the circulation and to direct albumin toward the circulation instead of to the bile (5).
FcRn at the blood-brain-barrier
The blood-brain-barrier (BBB) restricts access of large molecules to the central nervous system (CNS). Both IgG and albumin are excluded from the CNS and it has been suggested that FcRn mediates active transport of its ligands from the brain to the bloodstream (61, 62).
FcRn in hematopoietic cells
FcRn is highly abundant in cells of bone marrow in adult animals (63). It is expressed by monocytes, macrophages, neutrophils, DC and B lymphocytes but not T or natural killer cells (58, 60, 63-68). Although this still requires further investigation the presence of FcRn mainly in antigen presenting cells indicates it might provide functional benefits to these cells and directly implicates FcRn in IgG-mediated immune responses.

Our growing understanding of FcRn’s structure, ligand binding properties, expression patterns and biological function have led to the development of therapies that aim to either exploit FcRn binding or block it. Broadly speaking these can be divided into three categories: targeted delivery; half-life extension; or enhanced clearance.
There is a great desire to enable the non-invasive delivery of therapeutics across mucosal barriers and in addition to this, most communicable infections are initiated at mucosal sites. The role of FcRn in shuttling its ligands across the protective endothelial cell layer has thud led to the emergence of therapeutics aimed at enhancing transport of biologics across mucosal surfaces to improve drug absorption or distribution. Fusions to the IgG Fc domain have proven effective in nasal, pulmonary, oral, genital and in utero delivery of therapeutics or vaccines (69-78). Demonstration that albumin fusions, or albumin targeted binders, can be delivered across epithelial layers has not yet been established but albumin is present at high concentrations at mucosal surfaces and in extravascular spaces. It is also highly water soluble and stable, which would suggest it is a viable alternative to Fc fusions, especially where binding to classical FcγRs is undesirable.
Antibody based molecules have become the dominant format for biological therapeutics in a large part due to their interaction with FcRn which imparts a long-serum half life. This reduces dosing frequency and increases localization at the target site. Indeed, antibody engineering efforts have sought to further increase pH dependent binding of the Fc domain to FcRn to further enhance half-life by 2-5 fold (79-82). The ability of FcRn to extend half-life can also be exploited for non-antibody molecules that do not naturally contain an Fc domain by creating genetic fusions to the IgG Fc domain, albumin or an albumin binding domain. Like Fc engineering, albumin variants with enhanced binding to FcRn and improved half-life have been reported (83). Interestingly, clinical data is emerging to show that Fc fusions, and perhaps albumin fusions, may be more tolerogenic than their unfused counterparts (84-89).
Antibody engineering have also been developed for rapid degradation of target molecules, such as toxins or inflammatory cytokines, in a process typically referred to as antigen sweeping. Examples of such systems include acid or calcium switched antibodies (90) that dissociate from their antigen at acidic pH or at lower calcium concentrations which are found in endosomal vesicles. Such antibodies bind to their target antigen in the bloodstream and are taken up by cells. Once in the endosome the antibody disengages with its cargo. The IgG is recycled by FcRn while the antigen is trafficked to the lysosome for degradation. Unlike conventional antibody therapies this ensures the antigen half-life is limited while maintaining the long half-life of the therapeutic.
Lastly, blockade of FcRn can be used to alleviate IgG mediated autoimmune diseases by decreasing circulating levels of the auto-antibodies. Several strategies have been used, including engineering of antibodies with Fc regions that bind at both neutral and acidic pH, anti-FcRn antibodies that block the IgG binding site and FcRn inhibitory peptides and proteins (91-100).

1. Brambell, F. W. R. The Passive Immunity of the Young Mammal. Biological Reviews 33, 488–531 (1958).
2. Simister, N. E. & Rees, A. R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol 15, 733–738 (1985).
3. Raghavan, M. & Bjorkman, P. J. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol 12, 181–220 (1996).
4. Chaudhury, C. et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197, 315–322 (2003).
5. Pyzik, M. et al. The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol 10, 1540 (2019).
6. Burmeister, W. P., Gastinel, L. N., Simister, N. E., Blum, M. L. & Bjorkman, P. J. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature 372, 336–343 (1994).
7. Simister, N. E. & Mostov, K. E. An Fc receptor structurally related to MHC class I antigens. Nature 337, 184–187 (1989).
8. Story, C. M., Mikulska, J. E. & Simister, N. E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med 180, 2377–2381 (1994).
9. Andersen, J. T., Daba, M. B., Berntzen, G., Michaelsen, T. E. & Sandlie, I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem 285, 4826–4836 (2010).
10. Stapleton, N. M. et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun 2, 599 (2011).
11. Raghavan, M., Bonagura, V. R., Morrison, S. L. & Bjorkman, P. J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 34, 14649–14657 (1995).
12. Kim, J. K. et al. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol 29, 2819–2825 (1999).
13. Abdiche, Y. N. et al. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. MAbs 7, 331–343 (2015).
14. Wang, W. et al. Monoclonal Antibodies with Identical Fc Sequences Can Bind to FcRn Differentially with Pharmacokinetic Consequences. Drug Metab Dispos 39, 1469–1477 (2011).
15. Schlothauer, T. et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. MAbs 5, 576–586 (2013).
16. Piche-Nicholas, N. M. et al. Changes in complementarity-determining regions significantly alter IgG binding to the neonatal Fc receptor (FcRn) and pharmacokinetics. MAbs 10, 81–94 (2018).
17. Jensen, P. F. et al. Investigating the interaction between the neonatal Fc receptor and monoclonal antibody variants by hydrogen/deuterium exchange mass spectrometry. Mol Cell Proteomics 14, 148–161 (2015).
18. Schoch, A. et al. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc Natl Acad Sci U S A 112, 5997–6002 (2015).
19. Schmidt, M. M. et al. Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure 21, 1966–1978 (2013).
20. Andersen, J. T. et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun 3, 610 (2012).
21. Bern, M., Sand, K. M. K., Nilsen, J., Sandlie, I. & Andersen, J. T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J Control Release 211, 144–162 (2015).
22. Oganesyan, V. et al. Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem 289, 7812–7824 (2014).
23. Chaudhury, C., Brooks, C. L., Carter, D. C., Robinson, J. M. & Anderson, C. L. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry 45, 4983–4990 (2006).
24. Sand, K. M. K. et al. Interaction with both domain I and III of albumin is required for optimal pH-dependent binding to the neonatal Fc receptor (FcRn). J Biol Chem 289, 34583–34594 (2014).
25. Andersen, J. T. et al. Single-chain variable fragment albumin fusions bind the neonatal Fc receptor (FcRn) in a species-dependent manner: implications for in vivo half-life evaluation of albumin fusion therapeutics. J Biol Chem 288, 24277–24285 (2013).
26. Vaughn, D. E. & Bjorkman, P. J. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry 36, 9374–9380 (1997).
27. Firan, M. et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. International Immunology 13, 993–1002 (2001).
28. Zhou, J., Mateos, F., Ober, R. J. & Ward, E. S. Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J Mol Biol 345, 1071–1081 (2005).
29. Neuber, T. et al. Characterization and screening of IgG binding to the neonatal Fc receptor. MAbs 6, 928–942 (2014).
30. Grevys, A. et al. A human endothelial cell-based recycling assay for screening of FcRn targeted molecules. Nat Commun 9, 621 (2018).
31. Ober, R. J., Radu, C. G., Ghetie, V. & Ward, E. S. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol 13, 1551–1559 (2001).
32. Waldmann, T. A. & Strober, W. Metabolism of immunoglobulins. Prog Allergy 13, 1–110 (1969).
33. Ober, R. J., Martinez, C., Vaccaro, C., Zhou, J. & Ward, E. S. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol 172, 2021–2029 (2004).
34. Ward, E. S., Zhou, J., Ghetie, V. & Ober, R. J. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol 15, 187–195 (2003).
35. Weflen, A. W. et al. Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol Biol Cell 24, 2398–2405 (2013).
36. Jones, E. A. & Waldmann, T. A. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest 51, 2916–2927 (1972).
37. Rodewald, R. Intestinal transport of antibodies in the newborn rat. J Cell Biol 58, 189–211 (1973).
38. Wheeler, T. T., Hodgkinson, A. J., Prosser, C. G. & Davis, S. R. Immune components of colostrum and milk–a historical perspective. J Mammary Gland Biol Neoplasia 12, 237–247 (2007).
39. Cerutti, A., Chen, K. & Chorny, A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol 29, 273–293 (2011).
40. Claypool, S. M. et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fc gamma-receptor. Mol Biol Cell 15, 1746–1759 (2004).
41. Hornby, P. J. et al. Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis. Pharm Res 31, 908–922 (2014).
42. Yoshida, M. et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783 (2004).
43. Yoshida, M. et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 116, 2142–2151 (2006).
44. Muzammil, S. et al. FcRn binding is not sufficient for achieving systemic therapeutic levels of immunoglobulin G after oral delivery of enteric-coated capsules in cynomolgus macaques. Pharmacol Res Perspect 4, e00218 (2016).
45. Ben Suleiman, Y. et al. Neonatal Fc receptor for IgG (FcRn) expressed in the gastric epithelium regulates bacterial infection in mice. Mucosal Immunol 5, 87–98 (2012).
46. Armitage, C. W. et al. Divergent outcomes following transcytosis of IgG targeting intracellular and extracellular chlamydial antigens. Immunol Cell Biol 92, 417–426 (2014).
47. Brambell, F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 2, 1087–1093 (1966).
48. Malek, A., Sager, R. & Schneider, H. Maternal-fetal transport of immunoglobulin G and its subclasses during the third trimester of human pregnancy. Am J Reprod Immunol 32, 8–14 (1994).
49. Malek, A., Sager, R., Zakher, A. & Schneider, H. Transport of immunoglobulin G and its subclasses across the in vitro-perfused human placenta. Am J Obstet Gynecol 173, 760–767 (1995).
50. Einarsdottir, H. K. et al. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj J 30, 147–157 (2013).
51. Gitlin, D., Kumate, J., Urrusti, J. & Morales, C. The selectivity of the human placenta in the transfer of plasma proteins from mother to fetus. J Clin Invest 43, 1938–1951 (1964).
52. Lambot, N. et al. Evidence for a clathrin-mediated recycling of albumin in human term placenta. Biol Reprod 75, 90–97 (2006).
53. Haymann, J.-P. et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11, 632–639 (2000).
54. Akilesh, S. et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105, 967–972 (2008).
55. Sarav, M. et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20, 1941–1952 (2009).
56. Tenten, V. et al. Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24, 1966–1980 (2013).
57. Kobayashi, N. et al. FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol 282, F358-365 (2002).
58. Latvala, S., Jacobsen, B., Otteneder, M. B., Herrmann, A. & Kronenberg, S. Distribution of FcRn Across Species and Tissues. J Histochem Cytochem 65, 321–333 (2017).
59. Borvak, J. et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 10, 1289–1298 (1998).
60. Akilesh, S., Christianson, G. J., Roopenian, D. C. & Shaw, A. S. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 179, 4580–4588 (2007).
61. Zhang, Y. & Pardridge, W. M. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol 114, 168–172 (2001).
62. Cooper, P. R. et al. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res 1534, 13–21 (2013).
63. Zhu, X. et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol 166, 3266–3276 (2001).
64. Qiao, S.-W. et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A 105, 9337–9342 (2008).
65. Vidarsson, G. et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood 108, 3573–3579 (2006).
66. Mi, W. et al. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. J Immunol 181, 7550–7561 (2008).
67. Schneider, Z. et al. Overexpression of Bovine FcRn in Mice Enhances T-Dependent Immune Responses by Amplifying T Helper Cell Frequency and Germinal Center Enlargement in the Spleen. Front Immunol 6, 357 (2015).
68. Castaneda, D. C. et al. Lack of FcRn Impairs Natural Killer Cell Development and Functions in the Tumor Microenvironment. Front Immunol 9, 2259 (2018).
69. Bitonti, A. J. et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A 101, 9763–9768 (2004).
70. Gupta, N. et al. Regulation of immune responses to protein therapeutics by transplacental induction of T cell tolerance. Sci Transl Med 7, 275ra21 (2015).
71. Culina, S. et al. Materno-Fetal Transfer of Preproinsulin Through the Neonatal Fc Receptor Prevents Autoimmune Diabetes. Diabetes 64, 3532–3542 (2015).
72. Ye, L., Zeng, R., Bai, Y., Roopenian, D. C. & Zhu, X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol 29, 158–163 (2011).
73. Lu, L. et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol 85, 10542–10553 (2011).
74. Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med 5, 213ra167 (2013).
75. Grubb, J. H. et al. Infused Fc-tagged beta-glucuronidase crosses the placenta and produces clearance of storage in utero in mucopolysaccharidosis VII mice. Proc Natl Acad Sci U S A 105, 8375–8380 (2008).
76. Dumont, J. A. et al. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J Aerosol Med 18, 294–303 (2005).
77. Low, S. C., Nunes, S. L., Bitonti, A. J. & Dumont, J. A. Oral and pulmonary delivery of FSH-Fc fusion proteins via neonatal Fc receptor-mediated transcytosis. Hum Reprod 20, 1805–1813 (2005).
78. Vallee, S. et al. Pulmonary administration of interferon Beta-1a-fc fusion protein in non-human primates using an immunoglobulin transport pathway. J Interferon Cytokine Res 32, 178–184 (2012).
79. Hinton, P. R. et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem 279, 6213–6216 (2004).
80. Dall’Acqua, W. F. et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol 169, 5171–5180 (2002).
81. Dall’Acqua, W. F., Kiener, P. A. & Wu, H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 281, 23514–23524 (2006).
82. Lee, C.-H. et al. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat Commun 10, 5031 (2019).
83. Andersen, J. T. et al. Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem 289, 13492–13502 (2014).
84. Santagostino, E. et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood 127, 1761–1769 (2016).
85. Ragni, M. V., Alabek, M. & Malec, L. M. Inhibitor development in two cousins receiving full-length factor VIII (FVIII) and FVIII-Fc fusion protein. Haemophilia 22, e462-464 (2016).
86. Malec, L., Abshire, T., Jobe, S. & White, G. rFIXFc for Immune Tolerance Induction in a Severe Hemophilia B Patient with an Inhibitor and Prior History of ITI Related Nephrotic Syndrome. Haemophilia 24, e294–e296 (2018).
87. Carcao, M. et al. Recombinant factor VIII Fc fusion protein for immune tolerance induction in patients with severe haemophilia A with inhibitors-A retrospective analysis. Haemophilia 24, 245–252 (2018).
88. Kis-Toth, K. et al. Recombinant factor VIII Fc fusion protein drives regulatory macrophage polarization. Blood Adv 2, 2904–2916 (2018).
89. Groomes, C. L. et al. Reduction of Factor VIII Inhibitor Titers During Immune Tolerance Induction With Recombinant Factor VIII-Fc Fusion Protein. Pediatr Blood Cancer 63, 922–924 (2016).
90. Ward, E. S. & Ober, R. J. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol Sci 39, 892–904 (2018).
91. Liu, L. et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol 178, 5390–5398 (2007).
92. Low, S. C. & Mezo, A. R. Inhibitors of the FcRn:IgG protein-protein interaction. AAPS J 11, 432–434 (2009).
93. Nixon, A. E. et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front Immunol 6, 176 (2015).
94. Ulrichts, P. et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 128, 4372–4386 (2018).
95. Kiessling, P. et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med 9, eaan1208 (2017).
96. Ling, L. E. et al. M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study. Clin Pharmacol Ther 105, 1031–1039 (2019).
97. Patel, D. A. et al. Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J Immunol 187, 1015–1022 (2011).
98. Swiercz, R. et al. Use of Fc-Engineered Antibodies as Clearing Agents to Increase Contrast During PET. J Nucl Med 55, 1204–1207 (2014).
99. Vaccaro, C., Zhou, J., Ober, R. J. & Ward, E. S. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol 23, 1283–1288 (2005).
100. Seijsing, J., Yu, S., Frejd, F. Y., Höiden-Guthenberg, I. & Gräslund, T. In vivo depletion of serum IgG by an affibody molecule binding the neonatal Fc receptor. Sci Rep 8, 5141 (2018).